RESPIRATION IN PLANTS
INTRODUCTION
- Various cellular activities in living organisms like absorption, transport, muscle-contraction, locomotion, nerve-impulse conduction, reproduction, growth, development, seed germination or breathing require energy.
- All the energy required for 'life' processes in all living organisms comes from the oxidation of organic molecules.
- Only green plants and cyanobacteria (blue-green algae) can prepare their own food by the process of photosynthesis. In green plants, only cells containing chloroplasts carry out photosynthesis. Even in green plants all other organs, tissues and cells that are non-green, need food for oxidation.
- Animals obtain their food from plants directly (herbivores) or indirectly (carnivores). Saprophytes like fungi are dependent on dead and decaying matter for obtaining energy.
- Cellular respiration is an enzyme controlled process of biological oxidation of food materials in a living cell, using molecular O2, producing CO2 and H2O and releasing energy in gradual steps and storing it in biologically useful forms, generally ATP.
- So respiration is catabolic, exothermic and oxidative process.
- Most of the respiration processes occur in mitochondria.
- Respiratory substrates are compounds that are oxidised during the process of respiration. Usually, carbohydrates are oxidised to release energy but proteins, fats and even organic acids can be used as respiratory substances in some plants, under certain conditions.
- Energy trapped in ATP is utilised in various energy requiring processes of organisms, and the carbon compounds produced during respiration are used as precursors for biosynthesis of other molecules in the cell.
DO PLANTS BREATHE?
- Plants require O2 for respiration to occur and they also give out CO2. Hence, plants have systems in place that ensure the availability of O2. Plants, unlike animals, have no specialized organs for gaseous exchange but they have stomata and lenticels for this purpose.
- Plants get along without respiratory organs because each plant part takes care of its own gas exchange. There is little transport of gases from one part to another.
- Roots, stems and leaves respire at rates far lower than animals do. Only during photosynthesis, large volumes of gases are exchanged and, each leaf is well adapted to take care of its own needs during these periods.
TYPES OF RESPIRATION
On the basis of the availability of oxygen and the complete or incomplete oxidation of respiratory substrate, it is of two types :
- Aerobic respiration : When O2 is utilized during the process of respiration it is called aerobic respiration. In this process, there is complete oxidation of food and entire carbon is released as CO2 and large amount of energy is released.
- Anaerobic respiration : When there is no utilisation of O2 during respiration, then food substances are incompletely oxidized and produce alcohol or organic acids and most of the energy is lost in the form of heat.
Organisms can be grouped into the following four classes on the basis of their respiratory habit
- Obligate aerobes : These organisms can respire only in the presence of oxygen. Thus, oxygen is essential for their survival (e.g., bacterium Bacillus subtilis).
- Facultative anaerobes : Such organisms usually respire aerobically (i.e., in the presence of oxygen) but under certain conditions may also respire anaerobically (e.g., Yeast, parasites of the alimentary canal).
- Obligate anaerobes : These organisms normally respire anaerobically. Such organisms are in fact killed in the presence of substantial amounts of oxygen (e.g., Clostridium botulinum and C. tetani).
- Facultative aerobes : These are primarily anaerobic organisms but under certain conditions may also respire aerobically (e.g., yeast).
Table : Differences between Aerobic and Anaerobic respiration
Flow Chart : Types & Mechanism of cellular respiration.
GLYCOLYSIS
- All living organisms retain the enzymatic machinery to partially oxidise glucose without the help of oxygen. This breakdown of glucose to pyruvic acid is called glycolysis.
- The scheme of glycolysis was given by Gustav Embden, Otto Meyerhof and J. Parnas, and is often referred to as the EMP pathway.
- In anaerobic organisms, it is the only process of respiration.
- Glycolysis involves a series of ten biochemical reactions in cytoplasm.
- In plants, glucose is derived from sucrose, which is the end product of photosynthesis, or from storage carbohydrates. Sucrose is converted into glucose and fructose by the enzyme, invertase, and these two monosaccharides readily enter the glycolytic pathway.
- In glycolysis, neither consumption of oxygen nor liberation of CO2 takes place.
- In glycolysis, 1 glucose, produces 2 molecules of pyruvic acid (3C).
- In glycolysis, four molecules of ATP are formed by two ways:
- Direct / substrate phosphorylation of ADP to ATP.
- Another ATP is synthesized during the conversion of PEP to pyruvic acid.
- During aerobic respiration (when oxygen is available) each NADH2 forms 3 ATP and H2O through electron transport system of mitochondria. In this way during aerobic respiration there is additional gain of 6 ATP in glycolysis
- Glycolysis is also known as oxidative anabolism or catabolic resynthesis, because it is linked with anabolism of fats and amino acids. An intermediate phosphoglyceraldehyde (PGAL) is used for the synthesis of glycerol which later forms fats or lipids. PGA is used for synthesis of amino acids like serine, glycine, cystine. Alanine forms from pyruvate.
- Phosphofructokinase is an allosteric enzyme. The phosphorylation of fructose-6-phosphate is the most important regulation reaction of glycolysis.
- Phosphofructokinase has multiple allosteric modulators. It’s activity is inhibited by ATP (–ve modulator) and stimulated by ADP & AMP (+ve modulator).
- The end product of glycolysis are 2 molecules of pyruvic acid, NADH + H+, H2O and ATPs.
- Further oxidation of pyruvic acid and NADH2 after glycolysis in mitochondria requires oxygen.
- Pyruvic acid is the key product of glycolysis. The metabolic fate of pyruvate depends on the cellular need.
- Further fate of pyruvic acid depends upon the availability of O2 and one of the given three routes is followed
- Lactic acid fermentation
- Aerobic respiration
- Alcoholic fermentation
BIO-CHEMICAL REACTIONS OF GLYCOLYSIS
FERMENTATION
- Fermentation is the incomplete oxidation of glucose under anaerobic conditions, where pyruvic acid is converted to CO2 and ethanol.
- In micro-organisms the term anaerobic respiration is replaced by fermentation which is known after the name of its major products, e.g. alcohol fermentation, lactic acid fermentation.
- The enzymes, pyruvic acid decarboxylase and alcohol dehydrogenase catalyzes fermentation reactions. Other organisms like some bacteria produce lactic acid from pyruvic acid.
- In animal cells also, like muscles during exercise, when oxygen is inadequate for cellular respiration, pyruvic acid is reduced to lactic acid by lactate dehydrogenase.
- The reducing agent is NADH+H+ which is reoxidized to NAD+ in alcoholic and lactic acid fermentation.
Different types of fermentation are :
- Alcoholic fermentation : Buchner discovered the enzyme zymase complex, which is responsible for alcoholic fermentation. This is the oldest & the best known type of fermentation performed by yeast & some bacteria.
- Lactic acid fermentation : It occurs in lactic acid bacteria (Lactobacillus) and in muscles during exercise (human). Pyruvic acid produced in glycolysis is reduced by NADH2 to form lactic acid without producing carbon dioxide.
- Acetic acid fermentation : This is aerobic fermentation.
- Butyric acid fermentation : It helps in the processing of rancid butter and jute fibres.
AEROBIC RESPIRATION
The final product of glycolysis, pyruvate is transported from the cytoplasm into the mitochondria. The crucial events in aerobic respiration are :
- The complete oxidation of pyruvate by the stepwise removal of all the hydrogen atoms, leaving three molecules of CO2.
- The passing on of the electrons removed as part of the hydrogen atoms to molecular O2 with simultaneous synthesis of ATP.
Acetyl Co-A is formed in peri mitochondrial space by enzyme pyruvate dehydrogenase complex comprises of (Mg++, LA (Lipoic Acid), TPP (Thiamine pyrophosphate), NAD, CoA)
2 moles of Pyruvic acid + 2 Co-A 2 Acetyl Co-A + 2CO2
2 Acetyl Co-A + 2CO2
Acetyl Co-A is a connecting link between glycolysis & Krebs-cycle. Decarboxylation and dehydrogenation (oxidative decarboxylation) takes place during formation of acetyl Co-A.
KREBS' CYCLE / TCA (TRICARBOXYLIC ACID) CYCLE) / CITRIC ACID CYCLE
- Krebs cycle is also called the citric acid cycle after one of the participating compounds.
- All the enzymes, reactants, intermediates and products of TCA cycle are found in the matrix, except succinate dehydrogenase (mitochondrial marker enzyme) which is located in the inner mitochondrial membrane.
- The synthesis of GTP by the conversion of succinyl–CoA to succinic acid is a substrate level phosphorylation.
- 3NADH2, 1FADH2 & 1GTP (ATP) are produced by each turn of TCA cycle.
- One mole of acetyl CoA gives 12 ATPs during oxidation through Kreb's cycle.
Fig. : Diagramatic representation of oxidative decarboxylation of pyruvic acid and different chemical reactions in Kreb's cycle starting from Acetyl CoA
BIO-CHEMICAL REACTIONS IN KREBS CYCLE
- Citric acid
- Isocitrate + NAD+
- Cis-aconitic acid + H2O
Isocitric acid
- Oxalosuccinic acid
Succinyl CoA + CO2
Succinic acid + CoA.SH (Energy of thioester bond is released, which used in formation of GTP)
The GTP formed in reaction 7, reacts with ADP to form ATP and GDP, as GTP and ATP have approximately the same energy.
Fumaric acid (4C)
- Malic acid
The summary equation for this phase of respiration may be written as follows :
Pyruvic acid + 4NAD+ + FAD+ + 2H2O + ADP + Pi 3CO2 + 4NADH + 4H+ +
3CO2 + 4NADH + 4H+ +
FADH2 + ATP
Because of the decomposition of one molecule of glucose, 2 molecules of Acetyl CoA are formed. So, due to decomposition of 1 molecule of glucose, the cycle runs two times.
Total energy production in TCA cycle
DIFFERENCES BETWEEN GLYCOLYSIS AND KREB'S CYCLE
ELECTRON TRANSPORT SYSTEM (ETS) AND OXIDATIVE PHOSPHORYLATION
- The metabolic pathway through which the electrons passes from one carrier to another, is called the electron transport system and it is present in the inner mitochondrial membrane.
- The system consists of a series of precisely arranged nine electron carriers (coenzyme) in the inner membrane of the mitochondrion. These nine electron-carriers function in a specific sequence: Nicotinamide adenine dinucleotide (NAD), Flavin mononucleotide (FMN), Flavin adenine dinucleotide (FAD), Co-enzyme-Q or ubiquinone, Cytochrome-b, Cytochrome-c1, Cytochrome-c, Cytochrome-a and Cytochrome-a3.
- The ETC is comprised of four complexes and two mobile carriers i.e. coenzyme Q, a non protein part of the chain
- Complex I : Consists of flavoproteins of NADH dehydrogenase (FPN).
- Complex II : Consists of flavoproteins of succinic dehydrogenase.
- Between complexes II and III, is the mobile carrier-coenzyme Q (CoQ) or ubiquinone (UQ).
- Complexes III : Consists of cytochrome b and cytochrome c1. Associated with cytochrome b is the non-haeme iron of complex III (Fe NHR).
- Complex IV : Consists of cytochrome a and cytochrome a3 and bound copper that are required for this complex reaction to occur.
- The electrons either follow the pathway of complexes I, III and IV or II, III and IV.
- Electrons from NADH produced in the mitochondrial matrix during citric acid cycle are oxidized by an NADH dehydrogenase (complex I), and electrons are then transferred to ubiquinone located within the inner membrane.
- Ubiquinone also receives reducing equivalents via FADH2 generated during the oxidation of succinate by succinate dehydrogenase (complex II).
- The reduced ubiquinone, called ubiquinol, is then oxidized by transfer of electrons to cytochrome c, cytochrome bc1 – complex (complex III).
- Cytochrome c acts as a mobile carrier between complex III and complex IV.
- Complex IV refers to cytochrome c oxidase complex containing cytochromes a and a3 and two copper centres.
- When the electrons pass from one carrier to another carrier via complex I to IV in the electron transport chain, they are coupled to ATP synthase (complex V) for the formation of ATP from ADP and Pi.
- Oxygen functions as the terminal acceptor of electrons and is reduced to water along with the hydrogen atoms. It drives the whole process by removing hydrogen from the system.
- In respiration, energy of oxidation-reduction is utilized for the production of proton gradient.
- Higher proton concentration in the outer chamber causes the protons to pass inwardly into the matrix or inner chamber through the inner membrane.
- The energy of the proton gradient is used in attaching a phosphate radicle to ADP by high energy bond. So the process is called oxidative phosphorylation.
- Oxidation of one molecule of NADH2 produces 3 ATP molecules while a similar oxidation of FADH2 forms 2 ATP molecules.
- ATP synthase (complex V) helps in ATP synthesis. It consists of two major components F1 and F0. F1 (head piece) is a peripheral membrane protein complex and contains the site for ATP synthesis while F0 is an integral membrane protein complex that forms a channel through which protons cross the inner membrane. For each ATP produced, 2H+ passes through F0 from the intermembrane space to the matrix down the electrochemical proton gradient.
RESPIRATORY CHAIN INHIBITORS
- Rotenone : It checks flow of electrons from NADH / FADH2 to CoQ.
- Antimycin A : Transfer of electrons from Cyt b to Cyt c1 is prevented.
- Cyanide : It prevents flow of electrons from Cyt a3 to oxygen.
- Dinitrophenol (2, 4-DNP) : It prevents synthesis of ATP from ADP because it directs electrons from coQ to Q2.
ROLE OF SHUTTLE SYSTEM IN ENERGY PRODUCTION
Glycolysis occurs in the cytoplasm outside the mitochondrion in which 2NADH2 molecules are produced but ETC is located along the inner mitochondrial membrane, so NADH2 of glycolysis must enter inside the mitochondrion to release energy. But the inner mitochondrial membrane is impermeable to NADH2.
In mitochondrial membrane, there are 2 shuttle-systems, each formed of carrier-molecules. These shuttle system are - malate aspartate system and glycerol phosphate shuttle system.
- Malate-Aspartate shuttle : When this electron shuttle operates, transfer of electrons takes place from NADPH2
(in cytoplasm) to NAD inside the mitochondria. This is more efficient and results in production of 38 ATP molecules. - Glycerol-Phosphate shuttle : In this shuttle, electrons are transferred from NADH2 (in cytoplasm) to FAD (inside mitochondria). It results in production of 36 ATP molecules. It is less efficient and results in the reduction of FAD inside the mitochondrion.
GLYOXYLATE CYCLE
- Discovered by Kornberg & Kreb, during germination of fatty seeds.
- This cycle converts fats into sugars, so it is an example of gluconeogenesis in plants.
- Glyoxylate cycle occurs in glyoxysome, cytosol and mitochondria.
RESPIRATORY BALANCE SHEET
The calculations of net gain of ATP , for every glucose molecule oxidized, is made on certain assumptions that are as follows :
- There is a sequential, orderly pathway functioning, with one substrate forming the next with glycolysis, TCA cycle and ETS pathway following one after another.
- The NADH synthesized in glycolysis is transferred into the mitochondria and undergoes oxidative phosphorylation.
- Hence, there can be net gain of 36 ATP molecules during aerobic respiration of one molecule of glucose.
Table : Differences between Aerobic Respiration and Fermentation
AMPHIBOLIC PATHWAY
- Respiration involves the breakdown of organic compounds (glucose, pyruvate, acetyl co-A), so it has been considered as a catabolic process.
- Many amino-acids (α-ketoglutarate etc.) and fatty acids precursors are formed, so it is also an anabolic process.
- As it constitutes both catabolic and anabolic process, it is known as an amphibolic process.
Fig. : Inter-relationship among metabolic pathways showing respiration mediated breakdown of different organic molecules to CO2 and H2O
RESPIRATORY QUOTIENT (R.Q.)
The ratio of the volume of CO2 released to the volume of O2 taken in during respiration is called Respiratory Quotient (R.Q.)
R.Q. = 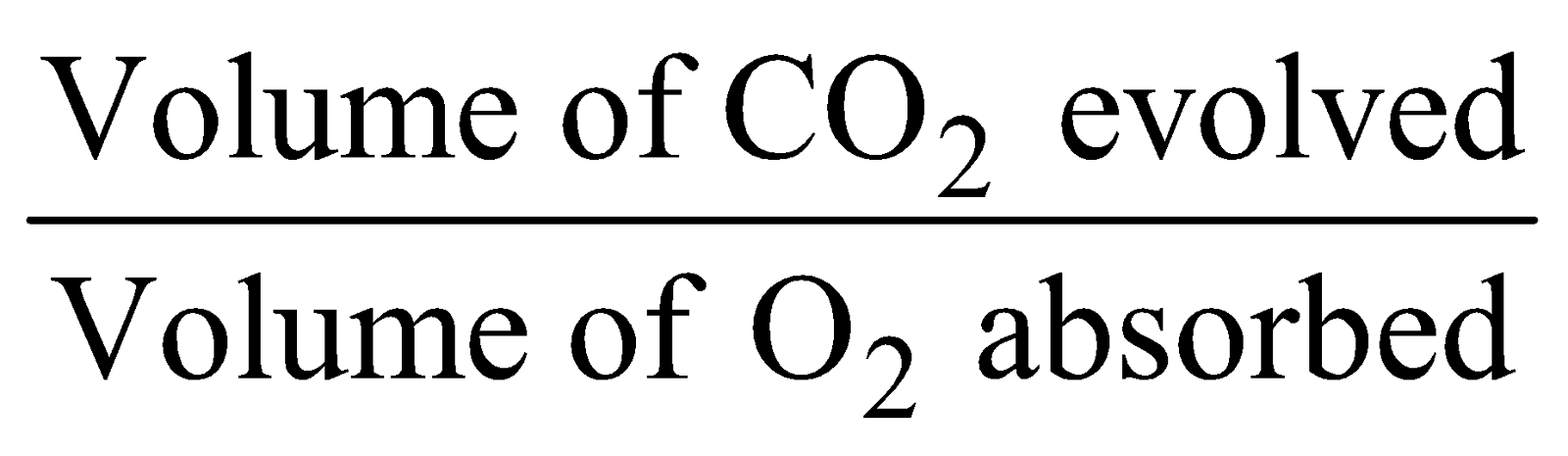
Value of R.Q. depends upon the nature of respiratory substrate used, amount of CO2 present in respiratory substrate, extent to which substrate is broken down, inter-conversion of one substrate, into another in the cell.
It is measured by Ganong's respirometer.
- Carbohydrates
C6H12O6 + 6O2 + 6H2O → 6CO2 + 12H2O + E
- Fat/Oil
2C51H98O6 + 145O2 → 102 CO2 + 98 H2O + E
- Organic acids
Oxalic acid, R.Q. = 4
Citric acid, R.Q. = 1.3
- Incomplete oxidation of carbohydrates (in the respiration of succulents i.e., Bryophyllum, Opuntia)
- Proteins
R.Q. = 0.8 or 0.9 or < 1 - Respiration in the absence of O2 (anaerobic respiration)
FACTORS AFFECTING THE RATE OF RESPIRATION
- Temperature :
- Optimum temperature for respiration is between 20-35°C. Maximum temperature is around 45°C.
- At low temperature, respiration is low due to inactivation of enzymes (refrigerator preserves food) while at very high temperature enzymes get denatured. Temperature coefficient Q10 = 2 to 2.5 for respiration.
- Oxygen : The inhibition of anaerobic respiration by increase in concentration of O2 is called as Pasteur's effect.
- CO2 : If CO2 concentration increases, then the rate of respiration decreases in plants because stomata get closed.
- Salts : If a plant is transferred from water to salt solution, it's respiration increases, this is known as salt respiration because absorption of ions requires metabolic energy.
- Hormones : IAA, GA and cytokinin increase the respiration rate.
The rapid increase in the rate of respiration during ripening of fruits and senescence of leaves and plant organs is called as "climacteric respiration". This rate decreases after sometime. It is due to production of ethylene hormone.
- Light : Rate of respiration increases with increase in light intensity. Light controls the stomatal opening and influence temperature and also produces respiratory substrates.
- Injury, disease & wounds : Respiration increases due to injury, wounding & infection.
- Age : Rate of respiration is more in young cells. Rate of respiration at meristem apex is high.
STRUCTURE OF ATP
- Nicotinamide adenine dinucleotide phosphate / Nicotinamide adenine dinucleotide (NADP/NAD) : It is called universal hydrogen acceptor, produced during aerobic respiration (glycolysis+ Kreb's cycle) and also in anaerobic respiration, works as a coenzyme in ATP generation via electron transport system. NADP has one additional phosphate.
- NAD plays a crucial role in dehydrogenation processes. Some dehydrogenases do not work with NAD, but react with NADP (Nicotinamide adenine dinucleotide phosphate). Nicotinamide is a vitamin of B group.
- Initially NAD and NADP both function as hydrogen acceptors. Later H+ ions and electrons (e_) from these are transported through a chain of carriers and after being released at the end of a chain, react with O2 and form H2O. During the release of 2 electrons from 2H+ atoms from NAD2H and their reaction with O2 to form water, 3 ATP molecules are synthesized from NAD or NADP.

إرسال تعليق